The following copyrighted
material is intended for individual use of the researcher, and may not be
copied or distributed without written permission from the copyright holder. You
may use it for non-profit scholarly purposes. Rivas,
J. A. and Levin, L. 2004. Sex
differential antipredator behavior in juvenile green iguanas, Iguana iguana: evidences for fraternal
care. pp: 119-126. In Behavior, Diversity, and Conservation of Iguanas (Alberts, A.
C., R. L. Carter, W. K. Hayes, and E. P. Martins, Eds.)
Sexually dimorphic anti-predator behavior in Juvenile green iguanas Iguana iguana: evidence for kin selection in the form of fraternal care.
By
Jesús A. Rivas and Luis E. Levín
Abstract: Animals that live in social groups may benefit from increased predator detection, deterrence and confusion. Juvenile green iguanas (Iguana iguana) are known to exhibit complex social behavior, often in sibling aggregations. Preliminary observations suggested that juvenile males and females performed different behaviors when facing predators. We conducted parallel lab and field experiments to study of the anti-predator behavior of juvenile green iguanas. First, we studied the behavior of juveniles in an enclosure when a model of a hawk was swung over them. Second, we measured the level of predation on males and females by free-ranging predators. Males showed more risk prone behavior when facing the simulated predator, and they also suffered higher predation by natural predators. Some males displayed a striking behavior that consisted of covering a female clutchmate with their body when the model hawk was diving over the enclosure. This behavior, in which a male assumes the predation risk of a female clutchmate, is the first report of fraternal care in a non-social vertebrate and may be related to the hierarchical social structure in green iguanas and the energetic contraints of herbivory.
Keywords: Iguana, kin selection, altruism, fraternal care, Antipredator behavior, herbivory.
Introduction
The benefits of sociality have been widely
discussed. Because the probability of detecting an approaching predator
increases with the number of guarding eyes, it has been proposed that animals
gain protection against predators by living in groups (Brown and Brown, 1987;
Da Silva and Therhune, 1988; Yáber and Herrera, 1994). Other benefits to
sociality include decreased likelihood of predation, selfish-herd effect (
Studies of anti-predator behavior in social reptiles have not been thorough in any species (Greene, 1988). In particular, studies of social behavior in iguanas have focused on territorial interactions and mating behavior (Alberts et al., 1992; Phillips et al 1993; Pratt et al., 1994; Rand and Rand, 1976; Rodda, 1992), although some attention has been given to the benefits of sociality in predator avoidance among green iguanas (Burghardt et al, 1977; Burghardt, 1977; see Burghardt this volume for a review). This studies have been observational rather than experimental.
Cooperation by relatives has been reported in
many species of social insects as a mechanism to increase fitness by increasing
the reproductive output of related individuals (
Initial observations
From 1988 to 1991 we incubated and hatched green iguanas from both natural and artificially incubated nests of green iguana (Iguana iguana) at Hato Masaguaral, a cattle ranch and biological field station located in Estado Guárico, Venezuela (8° 34' N, 67° 35' W). We repeatedly observed that when a researcher approached a naturally hatching nest, some animals remained immobile at the entrance to the nest, while one to several others fled. Such escape attempts were not usually directed toward cover, but, rather, toward the observer, or into relatively open space. The animals typically ran along a straight trajectory with their tails raised.
When handling iguanas incubated in artificial nests, they frequently ascended our arms, in an apparent escape maneuver potentially related to the natural tendency to escape by climbing trees. Similar to the behavior we observed in the field, other individuals either froze or hid in the bottom of the enclosure. Their subsequent behavior depended on the observer's behavior. Most remained immobile or hidden if we remained motionless, but if we chased the fugitive, then 5 or 6 additional individuals would flush from the nest. In all the all instances where we were able to capture the animal (n=7), it was a male. A similar behavior was observed in 10-month-old animals.
Given that the sex ratio at birth is1:1 (Rivas unpublished), we would expect to find females among the fugitives, if the likelihood of males and females exhibiting these behaviors were the same. Sexually dimorphic anti-predator behaviors have been reported in other juvenile squamata (Greene, 1988; Herzog et al., 1989).
Here we present two experiments that are designed to further explore sexual dimorphic anti-predator behavior. First, we compare the behavior of both sexes in response a model of a natural predator. Second, we examine escape behavior and measure mortality of both sexes when faced with free-ranging natural predators.
Experiment 1: Simulated predator
The first experiment was designed to study the behavior of juveniles facing a simulated predator. Field observations suggested that males reacted more actively than females to potential predators. However, since we did not know the sex ratio of the animals in the nest at the time we found them, we were unable to draw solid conclusions. In this experiment, we attempted to study anti-predator behavior of juvenile green iguanas under controlled conditions.
Methods
Five clutches of green iguanas were excavated and incubated independently in plastic buckets at ambient temperature (28 to 31 C° ). The newly hatched animals were transported to the laboratory at Universidad Central de Venezuela (10 30' N, 66 50' W). For five months, animals from different clutches were kept in separate outdoor enclosures measuring 60 x 60 x 80 cm and fed daily with a mixture of papaya and dog food supplemented with vitamins and minerals. Twelve experimental groups were formed, each composed of five females and five males from the same clutch. Sex was determined through visual cloacal examination (Rivas and Ávila, 1996).
The experimental arena was a rectangular opaque plastic enclosure measuring 180 x 30 x 30 cm. Dimensions were chosen to limit the direction in which the animals could flee, thus making it easier to score the behaviors. The bottom of the arena was lined with foam rubber to provide traction, with a refuge consisting of an opaque cover, 10 x10 cm, supported by four 2-cm legs at its corners, placed at the center of the arena. This refuge was. The refuge was encircled by a removable 25 x 25 x 30 cm transparent plastic corral, which at the beginning of the trial enclosed the iguanas (Figure 1A). The trial was initiated by remotely lifting the corral, to avoid disturbing the animals. The arena was illuminated by eight-reflector hood lights (75 W) hanging from the room ceiling.
The simulated predator consisted of a model of a hawk species (Falco femoralis), known to prey on juvenile iguanas (Rivas et al., 1999). The body of the model was constructed of wood and the wings of cardboard (39 cm long and 52 cm width). The shape, color and body pattern was taken from a descriptive illustration of the bird (Phelps and De Schauense 1978, Figures 1B). Eyes were simulated with two black dots (Gallup, 1973; Burger et al., 1991). In order to add a mechanical component to the stimulus, a celluloid sheet was hung below the model which, when in contact with the wall of the enclosure, produced noise and vibration. Thus, air movement, contact by the celluloid sheet (simulating the bird’s feathers) and the shadow of the falcon model (Prestude and Crawford, 1970) were additional components of the stimulus.
The model was fixed to one end of a pendulum. The other end was articulated at a hinge joint on the ceiling above the refuge. The pendulum was held horizontally by an electromagnet fixed to the ceiling of the room. When the electromagnet was turned off by an experimenter in an adjacent observation room, the model swept down over the arena.
All tests were carried out during the normal daily activity period. For ease of recognition during experimental trials, females were labeled with black tape on their backs and males with white tape. After two hours of acclimatization to the test arena, the plastic corral was removed, and five minutes later, the stimulus was presented. We let the model pass over the arena forward and backward three times and recorded the first movement performed by each animal during the three passages. After the study, all animals were returned to the field and released at the site where the eggs were collected.
The iguanas’ behavior was recorded with a video camera placed 2 m above the refuge. During preliminary trials, some iguanas concentrated at the end of the enclosure, and a second camera was directed at this area. Recording alternated between the two cameras by an electronic switch operating at 1-sec intervals. Trials were analyzed at 1/5 of real speed, and frame by frame, where necessary.
During the trial, the following mutually exclusive behaviors were scored during each passage of the pendulum: Move Ahead of the model running in the same direction as the model (MA), Move in the Opposite Direction of the model (MOD), Hide under the refuge (H), Appear from under the refuge and expose either part or all of the body (A). We also observed an unexpected behavior that consisted of one animal climbing onto another animal and covering it with its body at the moment when the model was starting its downward movement: Cover Another (CA). Animals that did not move at the end of the last passage where scored as Did Not Move (DNM).
Results and Discussion
We evaluated the effectiveness of the falcon model by comparing the behavioral responses of iguanas during the forward and backward movement of the pendulum. More animals responded actively to the normal (head forward) passes of the model than to the reverse (tail forward) passes (c 2 61.6, p<0.001;df= 1). Based on this we used only forward passages for the remaining of the analysis.
Risk-prone behaviors such as running in front of the hawk, appearing from under the cover, and covering another iguana were performed most often by males (Table 1). Females more often performed behaviors that increase safety including hiding, not moving, and running in the opposite direction of the model. Only males (7 times in the 12 trials) showed the behavior of covering another iguana, and it was always directed toward females. This behavior was never directed toward another male, nor was it ever performed by a female.
Because the expected frequencies of some of the behaviors presented in Table 1 are less than 5, we combined cells as follows before performing the statistical analyses (Siegel and Castellan 1988). Leaving the refuge (A) and covering another animal (CA) were lumped together as risk-prone behavior. Hiding (H) and to moving in the opposite direction (MOD), were lumped as risk adverse behaviors, since a flying hawk cannot stop in the air and fly backwards. The difference in the behavior of females and males in response to the model was highly significant (c2 = 24.43,) p < 0.001). In addition, the hypothesis of equal probability for both sexes of performing the behavior of covering another (CA) was rejected (p < 0.02) by a two-tailed binomial test.
Both male and female iguanas responded more strongly when the model predator passed in a forward direction. This result suggests that the iguanas discriminated the shape of the model, responding more actively to the head forward movement of the hawk, as found by Tinbergen (1948) in gray geese. The forward movement of the hawk also might have presented additional predator cues (e.g. eyes) to the iguanas than a backwards one (Gallup, 1973; Burger et al., 1991).
In evolutionary terms, the higher responsiveness of the males to the potential predator has two opposing (yet not mutually exclusive) potential explanations, one selfish and the other altruistic. First, this rapid response might surprise a searching predator and give the escapee more time to escape at the expense of the remaining animals. Alternatively, it might serve to divert the predator away from others, giving his relatives the opportunity to escape. If the fugitive escapes, he accomplishes the double goal of surviving and helping his clutchmates to escape. If he fails to escape, he may still provide the opportunity for his siblings to escape.
The selfish explanation requires that the escapee start the escape early, when the probability of escaping successfully is high. Conversely, the altruistic explanation requires that the escapee wait until the attack to the group is imminent and assumes that additional animals are present. Our data do not definitely discard either hypothesis. However, in our observations of natural nests, escapes took place after digging and harassing the animals for some time, such observations do not support so the selfish hypothesis. The high synchrony of hatching, where several hundred iguanas may emerge from a single nest site in approximately two or three weeks (Burghardt, 1977; Burghardt et al., 1977; Rivas et al., 1999) may limit the opportunities for predator learning. In fact, predators cueing on mass hatching events could have been an evolutionary force leading to such synchrony, as it has been documented in tadpoles of Bufo boreas that metamorphose synchronously to decrease predation by garter snakes (Devito et al., 1998).
Avoiding detection by predators is crucial for iguanas, given that a small iguana probably cannot repel a relatively large bird (Greene et al., 1978); one strategy to avoid being detected is to remain immobile (Prestude and Crawford, 1970). Greene et al. (1978) reported that a young iguana avoided being discovered by a hunting coati (Nasuasp.) by freezing. Our data shows that females stay motionless more often than males (Table 1).
Given the relatively high speed of an approaching falcon, to run in front of the predator is more likely to attract the hawk’s attention than to facilitate escape. Indeed, if an iguana attempted to escape by running, the best direction to run would be in the opposite direction of the flying hawk. Movements in the opposite direction of the model, which could have avoidance advantages, were more frequent in females than in males (Table 1).
Covering behavior is particularly striking. In all likelihood, a male that covers a female with his body increases his risk of being predated while decreasing hers. Our observations strongly suggest the existence of an altruistic behavior in which a male assumes the predation risk of his female clutchmates. Earlier studies have reported juvenile iguanas perching and sleeping in physical contact with each other and even on the top of each other (Burghardt, 1977; Burghardt et al., 1977 and pers. obs), indicating that the covering behavior is not likely to be an artifact of the experiment.
Experiment 2: Natural predation
The laboratory experiment showed that males are more risk-prone than females in their anti-predator behavior, a response pattern that could have opposing consequences for male survival. Males could surprise the predator, allowing them to escape more often than females who respond less actively. Another possibility is that the behavior of males attracts the attention of predators toward themselves, facilitating higher survival of their female cltuchmates. In this experiment we compared the survival probability of males and females facing free-ranging, natural predators.
Methods
This experiment was carried out at Hato Masaguaral. We excavated eight nests from the communal nest at the ranch (Rodda and Grajal, 1990) and incubated them until hatching. A total of 18 groups were used in the experimental trials. Each group was composed of 7 females and 7 males, from 0 to two weeks old, randomly chosen from the same clutch. Animals were identified with a number drawn with ink on the ventral side. Snout-vent length (SVL), total length (TL) and mass (W) were measured for each animal.
An outdoor escape-proof enclosure (3 x 3 m) was constructed with 60-cm-width zinc sheeting. Inside the enclosure was a shelter made from a wood board (40 x 40) cm on two cinder blocks under which food and water was placed. Several 40-cm natural bushes were included in the enclosure to provide natural perches and hides for the animals. Animals were released into the enclosure at 0600 hrs and exposed to natural predators until 1800 hr (12 trials) or released at 1800 hr and exposed to natural predators until 0600 hr (6 trails). At the end of each trial, we recorded which animals were present or absent. For the animals that were present, we noted which were missing a piece of the tail, as evidence of attack. Absent animals were scored as predated.
Results and Discussion
During diurnal trials we saw some avian predators flying nearby or perching next to the enclosure, including savanna hawks (Heterospizias meridionalis), crane hawks (Geranospizias caerulens), and greatkiskadees (Pitangus sulphuratus). A snake was also seen in the area (Chironius charinatus). All of these animals are known to prey on juvenile iguanas (Rivas et al 1999). Nocturnal predators seen included opossums (Didelphis marsupialis) and an unidentified rodent that entered the enclosure. Actual predation events could not be documented as our proximity deterred predators from approaching the enclosure.
The number of predated animals per trial varied from 1 to 14. Because this was a test without replacement, the likelihood of a given sex being predated decreased as the members of that sex were lost. Therefore, we excluded all the trials in which the number of predated animals was larger than five (8 replicates). Based on the remaining trails, we found that males were predated significantly more often than females (c2= 4.54, df= 1 p=0.03) (Table 2).
We compared the snout-vent length of males (74.89 ± 3.04 cm) with that of females (74.59 ± 2.57 cm) and found no significant difference (t= 0.63; df= 138; p= 0.53). There was no difference either when comparing the mass of males (12.44 ± 2.29 g) with females (12.45 ± 2.26 g; t = 0.04; df= 138; p = 0.97). Neonate green iguanas are not sexually dimorphic; therefore, predators selecting larger animals cannot explain the observed differences in predation rate between the sexes.
We also compared the size of animals that survived (74.89 ± 2.72 cm; 12.62 ± 2.26 g) versus those that were predated (74.61 ± 2.87cm; 11.93 ± 1.99 g). No significant effects were found in snout-vent length (t= 0.41; df = 138; p = 0.68) or mass (t = 1.32; df = 138; p = 0.19). Among the surviving animals, there were seven (five females and two males) that were missing a piece of the tail evidence that they had sustained an attack. The probability of being attacked (predated plus missing tail versus healthy animals) was not significantly different (c 2 = 1.61; df= 1; p> 0.2).
Our findings show that the high responsiveness of juvenile green iguanas to predators male does not seem to contribute to their individual survival. Males are predated more often than females, lending little support to the hypothesis that the males enhance their probability of successful escape by surprising the predators. Rather, he risk-prone behavior of males seems to attract the attention of natural predators toward them.
The refuge provided within the enclosure was large and protective enough for the iguanas to escape beneath and avoid detection. Thus, the animals that were predated had the option of either hiding of being exposed. The larger number of males predated cannot be explained by sexual dimorphism in body size, as we did not detect any differences between the sexes in SVL or mass between males and females nor between predated and not predated. Therefore, the higher number of males predated is presumed to be the result of behavioral differences, a conclusion supported by the observed differences in behavior between sexes in the laboratory experiment. The larger number of females missing part of the tail suggests that females do get attacked by predators but that they manage to escape predation more often than males. It is possible that some behavior such as CA by males toward females explains these findings, but further field observations are needed to document the extent to which there behavior patterns occur in nature.
General discussion
Male iguanas showed more risky-prone behavior than females in laboratory trials with the model predator, and were predated more often than females by natural predators. Here we offer two non-mutually exclusive explanations, one proximate, involving mechanism of control, and one ultimate, involving adaptive function, for our findings.
The risk-prone behavior of the males in the laboratory experiment could attract the attention of predators, resulting in the higher mortality that we observed in the field experiment. One possible explanation for this behavior in males is a consequence of the higher androgen levels, important for social dominance in early stages of maturation (Phillips et al., 1993; Pratt et al., 1994). To be dominant early may produce a larger pay off later in life that outweighs the cost of increased risk of predation. Thus, the higher risk incurred by males may be a by product of the social system of green iguanas, in which dominant males perform the vast majority of mating as adults (Dugan 1982; Rodda 1992). However, higher androgen levels do not explain the difference in the direction of the runs performed in response to the predator model in which males ran in front of the model more often, while females preferred to run in the opposite direction. Nor does it explain the covering behavior exhibited by males directed towards females. In addition, this explanation requires that the benefits of high androgen levels outweighs the increased predation risk. Neonate green iguanas suffer extremely high predation pressure by a large variety of predators (Rivas et al., 1999), so the benefits of increased androgens would have to be extremely high.
Another explanation for our results involves
adaptive reasons for the observed differences in behavior of males and females.
Males react more actively than females, which may attract the attention of a
predator and increase the chance for clutchmates to escape. This apparently
altruistic behavior can be explained in terms of kin selection (
Female green iguanas perform seasonal migrations to lay eggs in communal aggregations showing a high degree of phylopatry (Bock et al., 1985; Rodda and Grajal, 1990). These nesting aggregations are isolated from one another, leading to low levels of heterozygosity (Bock and McCraken 1988). This pattern suggests the possibility of breeding with relatives (Waldman and McKinnon 1993) and the likelihood that the relatedness among the hatchlings is higher than the expected 0.5, condition under which cooperative behaviors are more likely to evolve (Michod 1993). Thus a male that attracts a predator toward himself and saves several clutchmates might be increasing his indirect fitness. Such fraternal care could help account for the maintenance of sociality in juvenile green iguanas. Because, only animals within a cohesive social group will benefit from such risk-prone behavior, it pays for clutchmates to stay together.
A remaining question is why males should direct their altruistic behavior differentially toward females. The explanation could be derived from differential variability in the reproductive success of males and females and characteristics of the mating system. Iguanas breed in harems that are vigorously defended by dominant males (Dugan, 1982; Rodda, 1992). A male cannot gain control of a harem until he reaches an appropriate size to fight and win contests. Dugan (1982) suggests that a male needs six or seven years to reach the size in which he can defend a territory and even then, only a fraction of these males can successfully control a harem. Females, on the other hand, virtually all breed by their third year; but some start breeding as early as 1.5 years. Once they reach maturity, females breed annually (Rand and Bock 1992; Werner, 1991). Due to high rates of predation in the wild and strong intrasexual competition, the probability of a male reaching breeding size and controlling a harem is very low. Thus, a male that protects his female clutchmates might be increasing his inclusive fitness since the variance of female breeding success is much lower than that of males
How consistent the risk-prone behavior is along the animals life is yet unknown. Dominance relations are established early in life among males green iguanas (Phillips at al. 1993); however the relationship between these dominance and mating success in adulthood can only be speculated. If the same relationship carries to adulthood and it ends up determining mating success, one might expect that subordinate males that have a lower chance to mate, will be more likely to perform risk-prone behaviors since his chances of breeding are low anyway. On the other hand the same high levels of androgen that lead a neonate male to be dominant among his siblings might lead him to perform the risk-prone behaviors so this scenario is unlikely. Another alternative scenario is that if a male is consistently passive and does not perform risk-prone behaviors, he will benefit from the costly escape of altruistic males. He will also maximize energy intake and grow larger, reaching breeding size faster and more likely than males that are altruistic, hence the selfish males would produce more offspring than the altruistic ones. The reproduction of males and females related to the altruistic individuals must maintain the altruistic genes in the population. Males carrying altruistic genes and performing altruistic behaviors would have a high probability of never breeding but thanks to their investment their relatives will have better chances to survive.
The proposed explanations for the risk-prone behavior explaining it due to high levels of androgens and the other one explaining it based on the increase in the indirect fitness of the males are non-mutually exclusive; indeed, they are complementary. A higher amount of androgens in the males selected for dominance and hierarchy (Phillips et al. 1993: Pratt, 1994), would predispose them to a higher risk of predation; lowering the likelihood of ever reaching adult size. This would decrease even farther its likely hood of breeding. Once the male has a lower probability than the females to reach breeding size it would pay for him to benefit his sisters who have a higher likelihood of passing their genes to the next generation. This scenario resembles somewhat the situation that we find in sterile workers of the social insects where some individuals forfeit reproduction and spend their life nurturing and protecting their relatives. Future studies should address this fascinating possibility.
The altruistic behavior documented here seems likely to be a consequence of extreme variance in reproductive success among males. This variance in reproductive success may be due, first, to the very long period of time it takes for dominant males attain the large body size needed for successful territory defense and harem control. Larger size has been related to the evolution of the increased colon complexity that is needed to have high efficiency digesting the energy-poor diet that characterizes herbivory (Iverson, 1982). Second, males with slow growth rate as a consequence of the low nutritional value of the plant matter will remain small for extended periods with a low probability of breeding compared to larger (older) males (Pough, 1973; Rand, 1978).
This is the first report of altruistic behavior in any reptile excluding than parental care. However, the traits that favor its evolution of it are not unique to green iguanas. All iguanines are folivorous and most have similar hierarchical social structures and mating systems. Therefore, the potential exists for altruism to be present in other related taxa. However, the evolution of altruistic behavior might not be favored or might be constrained in some groups. Taxa living on islands (Amblyrhynchus, Brachylopus, Cyclura, and Conolophus) with lower predation pressure and smaller clutch size would not be predicted to exhibit the altruistic behaviors reported here. Similarly iguanines living on the mainland that have an insectivorous stage in their life cycle (Ctenosaura) may be less likely to evolve altruistic behavior because the cost of sociality in juveniles might be much higher due to competition for more limited food sources. Mainland species that are herbivorous throughout their lives would be good species to examine for the behaviors described here. In particular the genus Sauromalus meets the conditions and is genetically closely related to green iguanas (Site et al 1996). The insular species, Iguana delicatissima would be another interesting group to consider because it is very closely related to I. Iguana, but does not experience the putative environmental conditions expected to lead to altruism. More details on the phyllogeny as well as on the ontogeny, consistency, and variance of these altruistic behaviors, as well as in their relationship to ultimate reproductive success of those that perform and those that benefit from the behaviors described here are needed to fully understand the potential role of altruism in iguanine lizards. We have shown how anti-predator strategies of green iguana, as other types of behavior (Burghardt 1977) seem to be far more complex than earlier believed. This is the first report of fraternal care in a non-avian reptile. Behavior of reptiles has been considered as primitive and simple. It is time to start looking at the reptilian behavior with the same glass that we look at the behaviors of other groups.
ACKNOWLEDGEMENTS:
We thank Mr. Tomás Blohm for his hospitality and interest in this research. To, Juhani Ojasti, Gordon Burghardt, Stanley Rand, Samantha Messier, and Mark Waters for their helpful comments on the manuscript. This research was supported by Grants CDCH C.03.10.1981/92, CONICIT S1. 95-000-726, and by The Wildlife Conservation Society.
Literature cited
Alberts,
A. C., Sharp, T. R., Werner,
Alexander, R. D.,
Noonan, K. M. and B. J. Crespi. 1991. The evolution of eusociality. pp: 3-43 In:
Sherman, P. W, Jarvis G. U. and R. D Alexander. (Eds.), The biology of the
naked mole-rat.
Bock, B. C. and G. F. McCraken. 1988. Genetic structure and variability in the green iguana (Iguana iguana). Journal of Herpetology 22: 316-322.
Bock B. C., Rand A.
S. and G. M. Burghardt. 1985. Seasonal migration and nesting site fidelity in
the green iguana. pp: 435-443. In: Rankin M. A. (Ed.), Migration
mechanism in adaptive significance. University of Texas Marine Science
Institute,
Brown, C. R. and M. B. Brown. 1987. Group-living in cliff swallows as an advantage in avoiding predators. Behavioral Ecology and Sociobiology 21: 97-106.
Burger, J., Gochfeld, M. and B. Murray. 1991. Role of a predator’s eye size in risk perception by basking iguana, Ctenosaura similis. Animal Behaviour 42:471-476.
Burghardt, G. M. 1977. Of iguanas and dinosaurs: social behavior and communication in neonate reptiles. American Zoologist 17: 177-190.
Burghardt G. M., Greene, H. W. and A. S. Rand. 1977. Social behavior in hatchling green iguana: life at a reptile rookery. Science 195: 689-691.
Burghardt G. M. and A. S. Rand. 1985. Group size and growth rate in hatchling green iguanas (Iguana iguana). Behavioral Ecology and Sociobiology 18: 101-104.
Da Silva, J. and J.
M. Terhune.
Devito, J., Chivers, D.P., Kiesecker, J. M., Marco, A., Wildy, E. L.; A. R. Blaustein. 1998. The effects of snake predation on metamorphosis of western toads, Bufo boreas (Amphibia, Bufonidae). Ethology 104: 185-193.
Drummond H. and G.
M. Burghardt 1982. Orientation in dispersing hatchling green iguanas, Iguana
iguana. pp: 271-291. In: G. M. Burghardt and A. S. Rand (Eds.),
Iguanas of the world :Their Ecology, Behavior and Conservation. Noyes
Dugan B. 1982. The
mating behavior of the green iguana, Iguana iguana. pp: 320-339. In: G.
M. Burghardt and A. S. Rand (Eds.), Iguanas of the world :Their Ecology,
Behavior and Conservation. Noyes Plublications.
Ellis, H. I. and J. P. Ross. 1978. Field observations of cooling rates of Gálapagos land iguanas (Conolophus subcristatus). Comparative and Biochemical Physiology 59A: 205-209.
Gallup, G. 1973. Simulated predation and tonic immobility in Anolis carolinenesis. Copeia 1973:623-624.
Greene, H. W. 1988.
Antipredator mechanism in reptiles. pp: 1-152. In: Gans, C. and R. Huey
(Eds.), Biology of the Reptilia: Ecology Defense and Life history Vol
16.
Greene H., Burghardt G., Dugan B. y S. A. Rand. 1978 Predation and defensive behavior of green iguanas (Reptilia, Lacertilia, Iguanidae). Journal of Herpetology 12: 169-176.
Gross, M. R. and A. M. MacMillan. 1981. Predation and the evolution of colonial nesting in bluegill sunfish (Lepomis macrochirus). Behavioral Ecology and Sociobiology 8: 163-174.
Harris, D. M. 1982.
Phenology, growth, and survival of the green iguana, Iguana iguana,
in northern
Herzog, H. A., Bowers, B. B. and G. M. Burghardt. 1989. Development of antipredator response in snakes: IV. Interspecific and intraspecific differences in habituation of defensive behavior. Developmental Psychology 22: 489-508.
Iverson, J. B.
1982. Adaptations to herbivory in iguanines lizards. pp: 60- 76. In: G.
M. Burghardt and A. S. Rand (Eds.), Iguanas of the world :Their Ecology,
Behavior and Conservation. Noyes
Krakauer, D. C. 1995. Groups confuse predators by exploiting perceptual bottleneck: a connectionist model of the confusion effect. Behavioral Ecology and Sociobiology 36: 421-429.
Michod, R. E.
(1993). Inbreeding and the evolution of social behavior. pp. 74-96. In: Thornill,
N. W (Ed.) The Natural History of Inbreeding and Outbreeding.
Phelps, Jr. W. H.
and M. De Schauensee. 1978. A guide to the birds of
Phillips, J. A., Albert, A. C. and N. C. Pratt. 1993. Differential resource use, growth, and the ontogeny of social relationships in the green iguana. Physiology. and Behavior 53: 81-88.
Pough, F. H. 1973 Lizard energetics and diet. Ecology54: 835-844.
Pratt, N. C., Philips, J. A., A. C. Alberts and K. S. Bolda. 1994. Functional versus physiological puberty: an analysis of sexual bimaturism in the green iguana, Iguana iguana. Animal Behaviour 47: 1101-1114.
Prestude A. and F. Crawford. 1970. Tonic immobility in the lizard Iguana iguana . Animal Behaviour 18: 391-395.
Rand, A. S. 1968. A
nesting aggregation of iguanas. Copeia 1968: 552-561.
Rand A. S. 1978. Reptilian arboreal folivores.
pp:115-130. In: Montgomery, G. G. (Ed.) The Ecology of Arboreal
Folivores, Smithsonian Institution press
Rand, A. S. and B.
C. Bock. 1992. Size variation and survivorship in nesting green iguanas (Iguana
iguana) in
Rand, A. S., and W. M. Rand. 1976. Agonistic behavior in nesting iguanas: A stochastic analysis of dispute settlement dominated by the minimization of energy cost. Zoological Tierpsychology 40: 279-299.
Rodda, G. H. 1992. The mating behavior of Iguana iguana. Smithsonian Contribution to Zoology 534: 1-40.
Rodda G. H. and A.
Grajal. 1990 The nesting behavior of the green iguana, Iguana iguana, in
the llanos of
Siegel, S. and N.
J. Castellan. 1988. Nonparametrics statistics for the behavioral
sciences. Second edition. McGraw Hill.
Sites, J. W.,
Tinbergen, N. 1948. Social releasers and the experimental method required for their study. Willson Bulletin 60:6-51.
Waldman, B. and J.
S. McKinnon. 1993. Inbreeding and Outbreeding in Fishes, Amphibians, and
Reptiles. pp: 250-282. In: Thornill, N. W (Ed.) The Natural History of
Inbreeding and Outbreeding.
Warner, D. A. 1997. An overview on the evolution of the family Iguanidae. Journal of the International Iguana Society 6: 57-65.
Werner,
Werner,
Wilson, E. O. 1971.
The insects society. Harvard Univeristy Press.
Yáber, M. C. and E.
A. Herrera. 1994. Vigilance, group size and social status in capybaras. Animal
Behaviour. 48: 1301-1307.
Figure 1A test arena for simulated predator presentations to juvenile green iguanas.
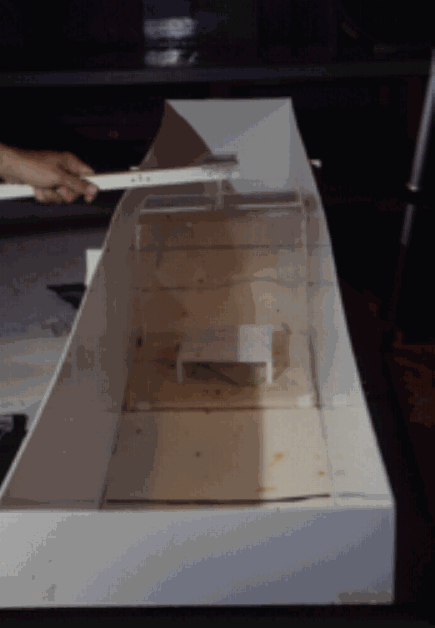
Figure 1B. Frontal view of the hawk model presented to juvenile green iguanas.

Table 1. Differential responses by male and female juvenile green iguanas to the forward passage of the model. Behaviors marked with a star are considered risk-prone behaviors.
|
|
Females |
Males |
|
Move ahead * |
20 |
35 |
|
Move in the opposite direction |
5 |
2 |
|
Hide |
6 |
3 |
|
Appear * |
1 |
4 |
|
Cover another * |
0 |
7 |
|
Did not move |
28 |
9 |
Table 2. Number of juvenile iguanas of each sex that were predated naturally in outdoor experimental enclosures. The predation rate for males was consistently equal of greater than that for females.
|
Trial |
Females |
Males |
|
1 |
0 |
2 |
|
2 |
2 |
2 |
|
3 |
0 |
1 |
|
4 |
0 |
2 |
|
5 |
1 |
0 |
|
6 |
1 |
1 |
|
7 |
0 |
1 |
|
8 |
0 |
2 |
|
9 |
1 |
3 |
|
10 |
1 |
1 |